Dr. Jörg Pabel
Department für Pharmazie
- Zentrum für Pharmaforschung -
Ludwig-Maximilians-Universität München
Butenandtstraße 7
D-81377 München
Zi.Nr.: C1.061
Tel.: +49 (0)89 2180 77244
Fax.: +49 (0)89 2180 77247
Mail: Joerg.Pabel@cup.lmu.de

Dissertation
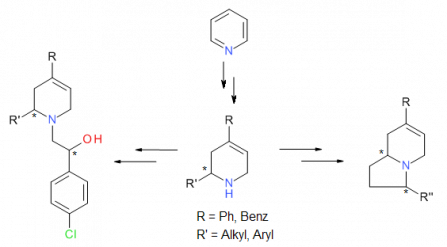
Asymmetrische Synthese von Antagonisten der Polyamin-Bindungsstelle des NMDA-Rezeptors
Membership

 Division VIII Advisory Subcommittee
Division VIII Advisory Subcommittee
Cover-Art
Publications
Ge, X.; Kasch, T.; Lewandowski, M.; Marschner, J.A.; Pabel, J.; Proschak, E.; Merk, D. Development of Potent and Selective Dual PPARδ/sEH Modulators From an AI-Designed Scaffold. J. Med. Chem., 2025, 68, 19, 20636–20656. DOI.
Jung, M.; Sałat, K.; Höfner, G.; Pabel, J.; Wyska, E.; Sierżęga, M.; Furgała-Wojas, A.; Gertzen, C.G.W.; Gohlke, H.; Wanner, K.T.; Synthesis and Biological Evaluation of Nipecotic Acid Derivatives with Terminally Double-Substituted Allenic Spacers as mGAT4 Inhibitors. J. Med. Chem., 2025, 68, 19, 19984–20010. DOI
Toews, S.; Dona, F.; Krauss, J.; Bracher, F.; López-García, Ú.; Pabel, J.; Merk, D.; Blommers, M.; Ferner, J.-P.; Wacker, A.; Richter, C.; Schwalbe, H. Targeting the SARS-CoV-2 RNA translation initiation element SL1 by molecules of low molecular weight. J. Am. Chem. Soc., 2025, , 28783-28798. DOI
Schallmayer, E.; Morozov, V.; Duensing-Kropp, S.; Schallmayer, L.; Schüffner, L.; Schubert-Zsilavecz, M.; Pabel, J.; Höfner, G.; Heering, J.; Marschner, J.A.; Merk, D. A First-in-Class Hepatocyte Nuclear Factor 4 Agonist. J. Med. Chem. 2025, 68, 10, 10410–10424. DOI
Nawa, F.; Sai, M.; Vietor, J.; Schwarzenbach, R.; Bitic, A.; Wolff, S.; Ildefeld, N.; Pabel, J.; Wein, T.; Marschner, J. A.; Heering, J.; Merk, D. Tuning RXR modulators for PGC1a recruitment. J. Med. Chem., 2024, 67, 18, 16338–16354. DOI
Isigkeit, L.; Hörmann, T.; Schallmayer, E.; Scholz, K.; Lillich, F.F.; Ehrler, J.H.M.; Hufnagel, B.; Büchner, J.; Marschner, J.A.; Pabel, J.; Proschak, E.; Merk, D. Automated design of multi-target ligands by generative deep learning. Nat. Commun., 2024, 15, 7946. DOI
Willems, S.; Busch, R.; Nawa, F.; Ballarotto, M.; Lillich, F.; Kasch, T.; López-García, Ú.; Marschner, J. A.; Rüger, L.; Renelt, B.; Ohrndorf, J.; Arifi, S.; Zaienne, D.; Proschak, E.; Pabel, J.; Merk, D. Structural Optimization of Oxaprozin for Selective Inverse Nurr1 Agonism. J. Med. Chem., 2024, 67, 13324-13348. DOI
Adouvi, G.; Nawa, F.; Ballarotto, M.; Rüger, L. A.; Knümann, L.; Kasch, T.; Arifi, S.; Schubert-Zsilavecz, M.; Willems, S.; Marschner, J. A.; Pabel, J.; Merk, D. Structural Fusion of Natural and Synthetic Ligand Features Boosts RXR Agonist Potency. J. Med. Chem., 2023, 66, 16762–16771. DOI
Sai, M.; Vietor, J.; Kornmayer, M.; Egner, M.; López-García, U.; Höfner, G.; Pabel, J.; Marschner, J.; Wein, T.; Merk, D. Structure-guided design of Nurr1 agonists derived from the natural ligand dihydroxyindole. J. Med. Chem., 2023. DOI
Vietor, J.; Gege, C.; Stiller, T.; Busch, R.; Schallmayer, E.; Kohlhof, H.; Höfner, G.; Pabel, J.; Marschner, J.; Merk, D. Development of a Potent Nurr1 Agonist Tool for In Vivo Applications. J. Med. Chem., 2023, 66, 6391–6402.
M. Daerr, J. Pabel, G. Höfner, P. Mayer, K. T. Wanner, "Synthesis and biological evaluation of fluorescent GAT-ligands based on meso-substituted BODIPY dyes", Med. Chem. Res. 2020, 29, 301–327 DOI.
T. Lutz, T. Wein, G. Höfner, J. Pabel, M. Eder, J. Dine, and K. T. Wanner, '"Development of new photoswitchable azobenzene based gamma-aminobutyric acid (GABA) uptake inhibitors with distinctly enhanced potency upon photoactivation", Journal of Medicinal Chemistry, 2018, 14, 6211-6235 (DOI).
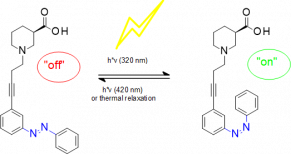
K. Salat, A. Podkowa, N. Malikowska, F. Kern, J. Pabel, E. Wojcieszak, K. Kulig, K.T. Wanner, B. Strach, E. Wyska, "Novel, highly potent and in vivo active inhibitor of GABA transporter subtype 1 with anticonvulsant, anxiolytic, antidepressant and antinociceptive properties", Neuropharmacology 2017, 113, 331-342 (DOI).
T. Wein, M. Petrera, L. Allmendinger, G. Höfner, J. Pabel, K. T. Wanner, "Different Binding Modes of Small and Large Binders of GAT1", ChemMedChem, 2016, 11, 509-518 (DOI).
M. Petrera, T. Wein, L. Allmendinger, M. Sindelar, J. Pabel, G. Höfner, K. T. Wanner, "Development of Highly Potent GAT1 Inhibitors: Synthesis of Nipecotic Acid Derivatives by Suzuki-Miyaura Cross-Coupling Reactions", ChemMedChem, 2016, 11, 519-538 (DOI).
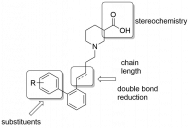
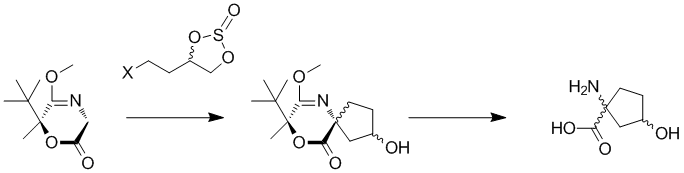
A. Jakubowska, J. Pabel, M. Żylewski, K. T. Wanner, K. Kulig, "Asymmetric synthesis of all four stereoisomers of 1-amino-3-hydroxy-cyclopentanecarboxylic acid", Tetrahedron 2015, 71, 686-693. (DOI).
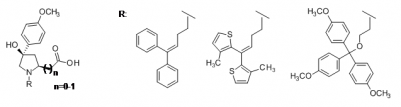
G. Quandt, G. Höfner, J. Pabel, J. Dine, M. Eder, K. T. Wanner, "First Photoswitchable Neurotransmitter Transporter Inhibitor: Light-induced Control of γ-Aminobutyric Acid Transporter 1 (GAT1) Activity in Mouse Brain", J.Med. Chem. 2014, 57, 6809–6821 (DOI)

X. Zhao, J. Pabel, G. Höfner, K.T. Wanner, "Synthesis and biological evaluation of 4- hydroxy-4-(4-methoxyphenyl)-substituted proline and pyrrolidin-2-ylacetic acid derivatives as GABA uptake inhibitors", Bioorg. Med. Chem., 2013, 2, 470–484. (DOI)
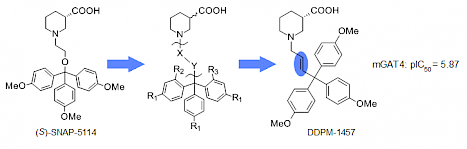
J. Pabel, M. Faust, C. Prehn, B. Wörlein, L. Allmendinger, G. Höfner, K. T. Wanner, "Development of a (S)-1-{2-[tris(4-methoxyphenyl)methoxy]ethyl}piperidine-3-carboxylic acid [(S)-SNAP-5114] carba analogue inhibitor for murine y- aminobutyric acid transporter type 4", ChemMedChem, 2012, 7, 1245 – 1255. (DOI)
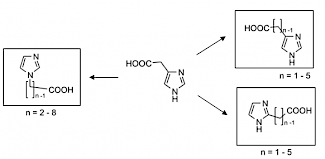
S. Hack, B. Wörlein, G. Höfner, J. Pabel, K.T. Wanner, "Development of imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors", Eur. J. Med. Chem., 2011, 46, 1483-1498. (DOI)
J. Bräckow, J. Pabel, P. Mayer, K. Polborn, K. T. Wanner, "Camphoric acid derived sulfur containing bicyclic carboxylic acids as chiral auxiliaries in N-acyliminium ion chemistry", Tetrahedron 2010, 36, 7279-7287. (DOI)
C. Schmaunz, J. Pabel, K.T. Wanner, "Synthesis of 5-substituted 7,8-benzomorphans by intramolecular cyclizations of N-protected 4,4-disubstituted 1,4-dihydropyridines", Synthesis, 2010, 13, 2147-2160. (DOI)
M.R. Faust, G. Höfner, J. Pabel, K.T. Wanner; "Azetidine Derivatives as Novel GABA Uptake Inhibitors: Synthesis, Biological Evaluation, and Structure-Activity Relationship", Eur. J. Med. Chem., 2010, 2453-2466. (DOI)
J. Pabel, E. Wadenstorfer, K.T. Wanner, "Asymmetric Synthesis of Pyridol[1,2-c]pyrimidones, Zeitschrift für Naturforschung B, 2009 (64b), 6, 653–661. (Link)
C.-J. Koch, S. Šimonyiová, J. Pabel, A. Kärtner, K. Polborn,K. T. Wanner, “Asymmetric Synthesis with 6-tert-Butyl-5-methoxy-6-methyl-3,6-dihydro-2H-1,4-oxazin-2-one as a New Chiral Glycine Equivalent: Preparation of Enantiomerically Pure α-Tertiary and α-Quaternary α-Amino Acids”, Eur. J. Org. Chem., 2003, 1244-1263. (DOI)
C. E. Hoesl, J. Pabel, K. Polborn, K.T. Wanner, „Synthesis of Sterically Demanding 3-Silylpyridines and their Use in Asymmetric Synthesis with Chiral N-Acyliminium Ions“, Heterocycles, 2002, 58, 383-392.(DOI)
C. E. Hoesl, M. Maurus, J. Pabel, K. Polborn, K. T. Wanner, “Generation of chiral N-acylpyridinium ions by means of silyl triflates and their diastereoselective trapping reaction: Formation of N-acyldihydropyridines and N-acyldihydropyridones”, Tetrahedron, 2002, 58, 6757-6770.(DOI)
J. Pabel, C. E. Hösl, M. Maurus, M. Ege, K. T. Wanner, "Generation of N-Acylpyridinium Ions from Pivaloyl Chloride and Pyridine Derivatives by Means of Silyl Triflates", J. Org. Chem., 2000, 65, 9272-9275. (DOI)
J. Pabel, G. Höfner, K. T. Wanner, "Synthesis and Resolution of Racemic Eliprodil and Evaluation of the Enantiomers of Eliprodil as NMDA Receptor Antagonists", Bioorg. Med. Chem. Lett.,2000, 10, 1377-1380.(DOI)





